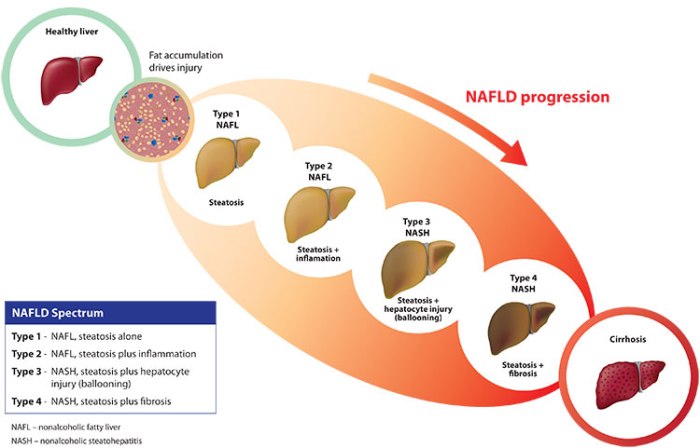
NASH is the acronym for nonalcoholic steatohepatitis. It’s a type of advanced liver disease that is clinically indistinguishable from alcohol-induced liver damage, but whose causes are totally different. Late stage NASH leads invariably to chronic cirrhosis and ultimately liver failure and death. The most common cause of NASH is long-term obesity, and so the threat has grown in relation to the rising rates of obesity in western society. Therefore NASH is quickly becoming an epidemic, with estimates of 15 to 30 million Americans living with the disease, the vast majority of whom are undiagnosed. They are living with a ticking time bomb inside their bodies. Continue reading “What is NASH?”
Liverfast offers a quick and non-invasive blood test to identify nafld/nash

One of the biggest challenges in tackling the NAFLD/NASH epidemic is that there has traditionally been no easy non-invasive way to detect it in patients. This is a large reason why there are tens of millions of people in the United States alone that are impacted but do not even know it.
That has changed significantly in the last year as a company called Fibronastics has released widely a simple blood screening test that can be used to detect levels of steatosis, inflammation activity, and fibrosis. The LIVERFASt test uses a machine learning algorithm to examine 10 separate biomarkers from the blood draw and uses a scoring system to provide results which normally cannot be identified without a biopsy.
This means that physicians now have a powerful non-invasive tool to help screen for liver illnesses or gauge the severity of diagnosed liver conditions without biopsy (huge!). Fibronastics has recently announced a partnership with The American Liver Foundation to provide free LIVERFASt tests for individuals who qualify, and most insurance companies as well as Medicare will now cover the tests as well.
The accuracy of the test has been well established with medical studies going back to 2019 concluding that it is both “reliable and reproducible”. The scoring system is easy-to-understand and arms patients with clear & concise information to utilize over the course of their journey with liver disease.

“It is a reliable, and reproducible tool which provides grading or staging of the three elementary features of NASH: steatosis, inflammatory activity and fibrosis”
As biopharma continues to search for effective medical treatments, LIVERFASt will become a critical tool in the early detection of NAFLD & NASH.
The Covid-19 Vaccine & Liver Disease
Many people living with Liver Disease have questions about the Covid-19 vaccine, including how effective it is and if there are any risks associated with it specifically related to Liver Disease. This article will attempt to summarize some of the publicly available information on vaccine efficacy and safety for those with Liver Disease. As always, you should consult with your doctor if you have specific questions or concerns.
Continue reading “The Covid-19 Vaccine & Liver Disease”FDA declines to approve Intercept’s Ocaliva
Today the FDA announced that it had declined to approve Ocaliva for treatment of NASH. Intercept Pharmaceuticals was set to be the first FDA approved NASH treatment, despite relatively mediocre Phase 3 Results announced in late 2019. This is a major disappointment for the tens of millions of NASH patients that were hoping for an imminent treatment to be available.
Continue reading “FDA declines to approve Intercept’s Ocaliva”Obesity is a Major Risk Factor in Covid-19 Cases – Perhaps the Largest

Strong evidence that obesity is a major risk factor in Covid-19 severity and poses an increased risk of death has now been demonstrated across multiple studies around the world. The evidence all points to obesity as the preexisting condition with the largest impact on unfavorable outcomes, across all age groups.
The data is especially striking for younger adults, long thought to be at a much lower risk for serious illness from Covid-19. One NYU Langone study of 3,615 Covid patients under 60 years of age showed that “Patients with a BMI of 30-34 were twice as likely to get admitted to the hospital or to be admitted to acute care. Patients with a BMI of 35 or higher were twice as likely to be admitted to the hospital, and three times as likely to end up in the intensive care unit.”
A CDC study found that of its patients, “89% had at least one underlying condition, with obesity being the most common for those between 18 and 64.” A Chinese study showed that obesity tripled the risk of a severe case versus those of normal weights.
Data from UK hospitalizations is even more stark, showing that obesity and metabolic syndrome are associated with a 10 times increase in death. In an article entitled “Covid 19 and the elephant in the room”, Dr. Aseem Malhotra states it bluntly; “OBESITY, THE REAL KILLER BEHIND COVID”
72.7% of patients admitted to ICU are overweight or obese and that those with the related metabolic syndrome have a tenfold increase in mortality from the virus.
Dr. Asseem Malhotra, NHS
Data from a set of studies in France recently published in The Lancet looked at the percentage of ICU patients with Covid that were obese versus the percentage of non-Covid ICU patients. It found a clearly higher rate of ICU admissions among obese patients for Covid vs other causes. This research is also anecdotally supported out of the NYC hospital data.
This blog has discussed the Obesity Epidemic many times before, and I’ve started examining some of the causes of it that are easier to control for. It was always clear that obesity & metabolic syndrome are important for far more than NASH, but this pandemic should serve as a clear wake-up call for the tens of millions of obese individuals around the world. We cannot hide from our health behind the well-intentioned shield of the Body Positivity movement. The warnings are now being shouted loud and clear:
“[P]eople with a body mass index of 30 or more should be taking the same precautions as someone over 65, regardless of their age.”
Dr. Carlos Galvani, Tulane Bariatric Center
Genfit Phase III NASH trial ends in failure
Last night French biotech company Genfit released the results of their widely anticipated Phase III study on Elafibranor, a NASH therapeutic that had shown tremendous promise in earlier trials.
Unfortunately, the trial failed to meet both the primary and secondary endpoints. The treated patients response wasn’t statistically meaningful versus the placebo control group. Genfit’s CEO Pascal Prigent said “These results are highly disappointing.” The stock is down by over 65% today.
This setback leaves us with just Abbvie’s cenicriviroc study to look forward to this year, due in late 2020 if there are no delays due to the Covid crisis. Intercept’s Ocaliva is due for FDA approval soon, but seems to have limited effectiveness in specific NASH cases.
The best treatment for NASH and NAFLD remains a healthy diet and lifetsytle changes for the foreseeable future.
NASH: 2020 Outlook

Around this time last year there were a number of news articles declaring 2019 “The Year of NASH” . Four companies were due to release Phase 3 trial results. Optimism was surging and awareness was beginning to catch on. So how did 2019 turn out, and what does the outlook for NASH in 2020 look like?
A year later:
- Gilead had several Phase 3 studies end that failed to live up to expectations, eliminating Selonsertib as a serious treatment option
- Intercept released mixed results for Ocaliva, but is pushing ahead for FDA Approval. If granted, it would be the first approved NASH treatement. Decision on approval should come after April 2020.
- Abbvie (acquired Allergan) pushed their Phase 3 results for cenicriviroc from 2019 to late 2020.
- Genfit delayed Phase 3 elafibranor results to Q1 2020. It remains a promising undercard.
So while we didn’t get any truly amazing results for treatment in 2019, we did make progress. The 2nd International NASH Day helped reach people across the globe. Awareness has been steadily growing.
And while we have yet to hear from Genfit on their treatment data, they did recently announce tremendeous progress in their quest for a non-invasive diagnostic test. Using a unique 4-biomarker algorithm they have been able to accurately identify NASH and significant fibrosis. Genfit plans to file for FDA approval of the test in 2020.
A reliable non-invasive test for NASH is in some ways even more important than a medical treatment. The danger from NASH comes from years of undiagnosed progression, and we already know that the liver damage NASH can cause is reversible in earlier stages of the disease.
The Stigma of Liver Disease
I do a lot of volunteer work with the American Liver Foundation. This frequently puts me in social settings at food & drink events where, upon hearing the name of the organization, I’m immediately confronted with the biggest stigma of Liver Disease. “Isn’t it a little odd to be talking about liver disease while holding a beer?”
I love these conversations, because the juxtaposition creates a teachable moment that is more likely than not to be remembered.
My response blows up their preconceptions. “Actually, there are over one-hundred types of liver disease that have nothing to do with alcohol. NASH is a serious progressive liver disease affecting over twenty million American’s alone”.
Continue reading “The Stigma of Liver Disease”The Best Diagnostic Tool for NASH
One of the questions I see asked all of the time is what kind of testing is the best to determine if someone has NASH. I’ve written about the challenge of diagnosis before, but there are a lot of scared individuals out there trying to get answers from their under-educated primary case physicians, and everyone wants to skip the preliminary steps and go straight to a definitive diagnosis. If only it were that easy!
Continue reading “The Best Diagnostic Tool for NASH”Watch the ALF NASH Day Webinar I participated in

For the 2nd International NASH Day yesterday I took part in a webinar along with Dr. Tuan Pham of the University of Utah. Dr. Pham gave a great overview of the latest in the NASH space and I shared the story of my mom to provide an advocate perspective.
You can view the video here:
Watch all of these amazing stories of NASH patients and providers
I recently posted my American Liver Foundation Advocacy video where I told Mom’s tragic story. Along with myself, several other patients currently living with various stages of NASH plus several medical professionals took part in the advocacy campaign.
The videos are now all available on the ALF Youtube page. Below are the embedded Youtube Playlists. Watch and share!